Kjeldahl's nitrogen determination may be the most popular method for determining nitrogen in organic substances, but it is not the only reference method in nitrogen analysis: In 1831, 52 years earlier, the French chemist Jean-Baptiste Dumas published his combustion method for quantitative nitrogen and protein determination.
The Dumas method is an elemental analysis for nitrogen determination and it is globally recognised and validated by international organisations such as AOAC, AACC, ISO, DIN, ASBC, AOCS or OIV.
In addition to the organic nitrogen content, the combustion method can also be used to analyse the total nitrogen content including inorganic components. Therefore, the Dumas method can also be used to determine nitrite (NO2) and nitrate (NO3), for example. The fact that inorganic components can also be analysed makes the Dumas nitrogen analysis particularly interesting for the food and animal feed industry because the demands on the composition of food and animal feed are constantly growing and detailed analytics are more important than ever for quality control and nutrition labelling.
Furthermore, the short analysis time, the simple operation and the high safety of the automated Dumas method are of great advantage, because a high sample throughput with a low expenditure of time is indispensable for most industrial sectors nowadays. Automated laboratory equipment for performing the Dumas method is therefore becoming increasingly popular not only in the food and feed industry, but also in areas such as environmental analytics. So, whether you need to analyse fertilisers in agriculture, whey products in the food industry or sustainable trends like insect protein: the Dumas method is versatile and delivers reliable results.
The manual nitrogen determination according to Dumas is very time-consuming because it is challenging to reproduce the conditions for the analysis. Thanks to advanced technology, however, modern combustion devices are fully automated. Thus, the Dumas method can now be integrated safely and easily into everyday laboratory work. Fully automated analytical systems such as the N-Realyzeruse the basic principles of the Dumas method for the determination of nitrogen and protein content in solid and liquid samples.
The combustion analysis according to Dumas
The traditional combustion method according to Dumas is used for the determination of the nitrogen content of combustible, mostly organic samples. The basic principle of this traditional method is still the foundation of modern, automated analysis systems today.
The classic Dumas method proceeds as follows: the sample is mixed with copper and then heated up at high temperatures with the addition of oxygen (O2). The result of that first step is a gas mixture. By adding copper to this gas mixture, it oxidises into the by-products water (H2O), carbon dioxide and nitrogen oxides (NOx). In the next step, the nitrogen oxides are reduced to elemental nitrogen (N2) via a copper wire. The N2 then passes through a potassium hydroxide solution and is collected in a graduated cylinder. The by-products carbon dioxide and water are separated.
Following this process, the nitrogen content can be read quantitatively and the nitrogen content of the sample can be calculated.
The automation of the Dumas Method
The steadily advancing automation of laboratory equipment has not left the Dumas method untouched: today, we have fully automated analysis systems that can carry out a nitrogen determination according to Dumas almost completely independently. This makes the work situation much easier for the laboratory staff, not only in terms of time, but also in terms of safety.
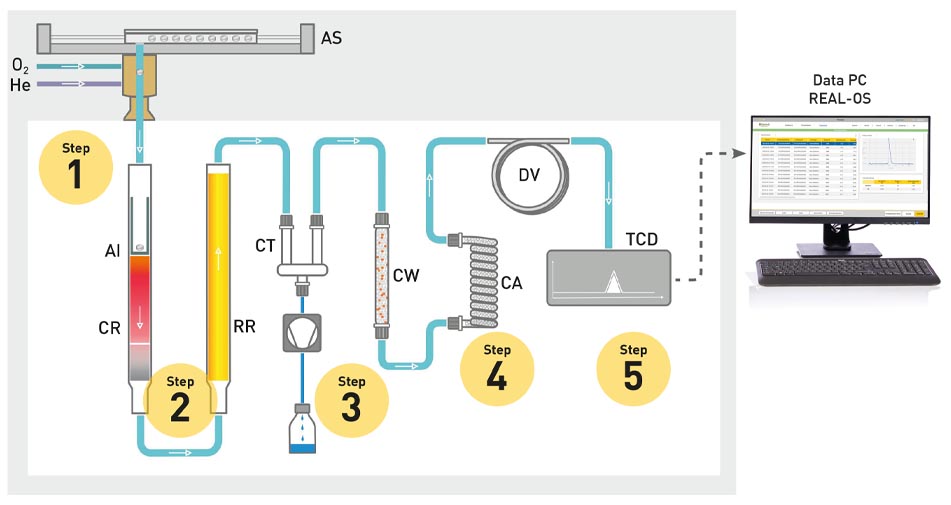
The analytical procedures have changed slightly with these modern systems, but still use the basics of the Dumas method:
The homogenised sample is combusted in a high-temperature furnace at approx. 1000 °C with the addition of pure oxygen (O2) (step 1). The resulting gas mixture, consisting of water, carbon dioxide, nitrogen oxides and nitrogen, is passed on through a carrier gas - usually helium. In the process, the nitrogen oxides are reduced to elemental nitrogen on a copper surface (step 2) and the water and carbon dioxide are separated (step 3 and 4). During the analysis, the carrier gas flows through the entire system and is constantly measured with a thermal conductivity detector (TCD) (step 5). At the end of the analysis, however, the TCD then measures a mixture of carrier gas and N2. This difference in gas composition creates a measurable voltage difference for the TCD, which is then used to calculate the nitrogen content of the sample.